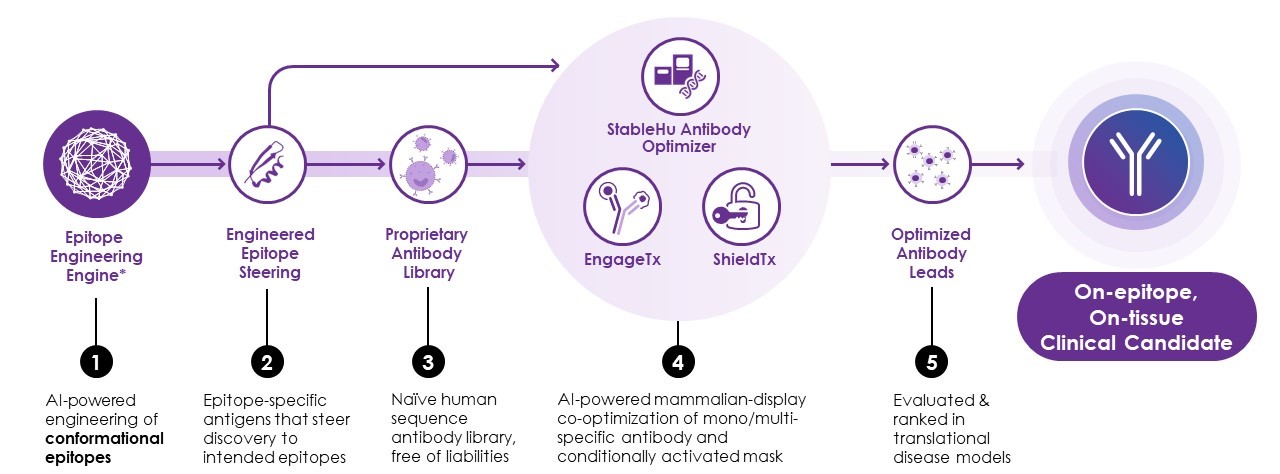
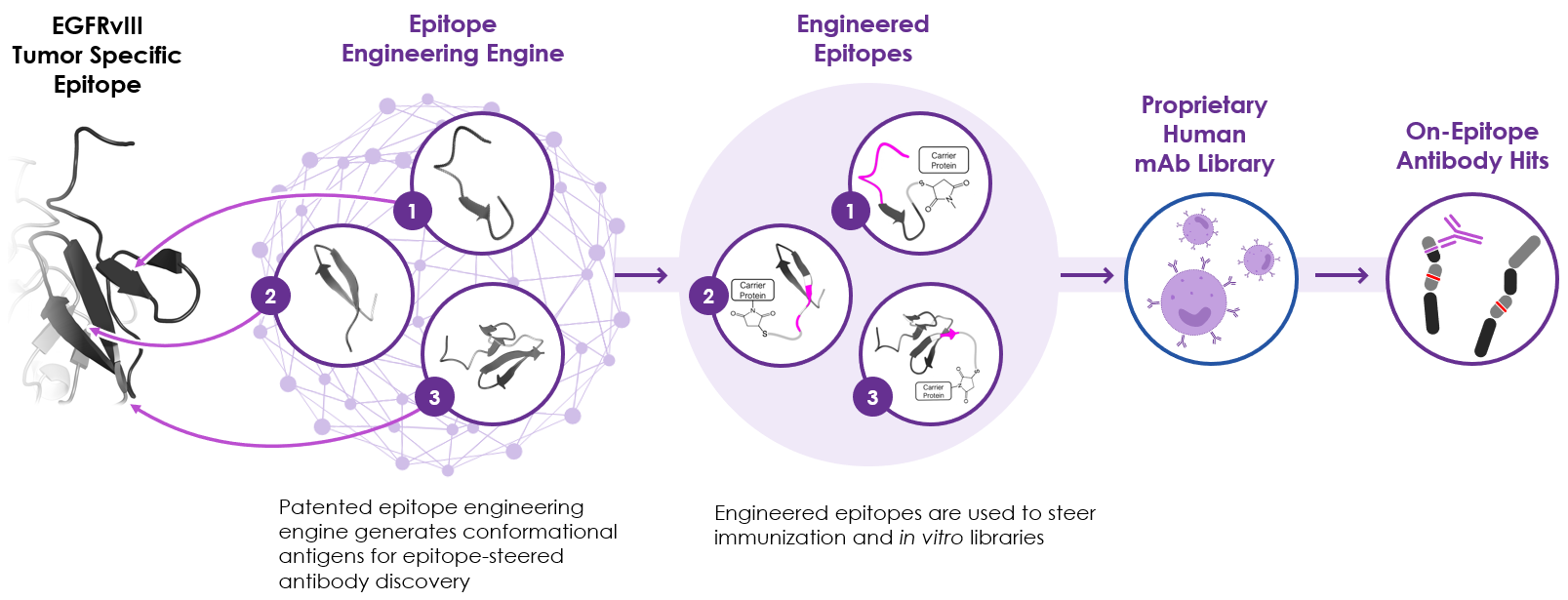
iBio’s Drug Discovery Platform2 aims to increase the likelihood of success. Using artificial intelligence (AI), our platform efficiently and consistently delivers antibody candidates against difficult targets.
At the heart of the platform is our patented epitope-engineering technology, which we use to engineer mini-proteins embodying the structure of conformational epitopes, including subdominant epitopes, to discover traditionally challenging therapeutic antibodies. An epitope is the region of a target protein to which antibodies bind. The range of potential epitopes or binding sites on a target is enormous. But not all epitopes are created equally.
Traditional drug discovery has allowed the immune system to drive epitope selection and thus biasing antibody selection towards immunodominant epitopes rather than subdominant epitopes. Often, the most efficacious epitopes are not immunodominant epitopes, but rather subdominant epitopes and therefore without epitope steering the best therapeutic antibodies are missed using traditional drug discovery methods.